| Synonyms: | COLO-678(人結(jié)腸癌細(xì)胞)Colo-678; COLO-678; COLO678 |
| Background: | Established from a metastatic lymph node of a 69-year-old man with colon carcinoma in 1984 |
| Species: | Homo sapiens, human |
| Tissue: | Colon |
| Disease: | Colorectal adenocarcinoma, derived from metastatic site: Lymph node. |
| Gender: | Male, 69 years |
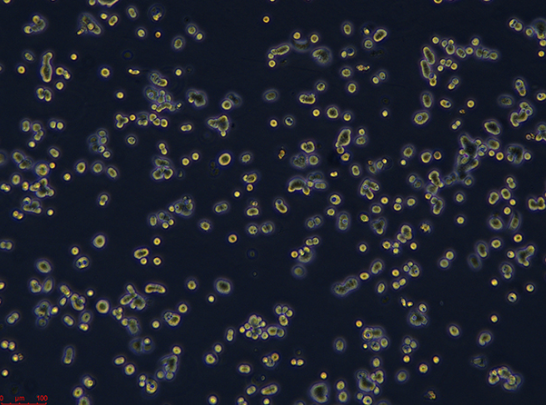
| Morphology: | Round or spindle-shaped adherent cells growing as monolayer |
| Growth Mode: | Adherent |
| Doubling Time: | 60~70hrs |
| DNA Profile: | Amelogenin: X |
| Culture Medium: | RPMI-1640+10%FBS |
| Cryopreservation medium: | 90% Complete Medium+10%DMSO |
| Photo: | NA |
| Comments: | Immunology: cytokeratin +, cytokeratin-7 -, cytokeratin-8 +, cytokeratin-17 -, cytokeratin-18 +, cytokeratin-19 +, desmin -, endothel -, EpCAM +, GFAP -, neurofilament -, vimentin – |
II. Handling Procedure for Flask Cultures
| The flask was seeded with cells grown and completely filled with medium at to prevent loss of cells during shipping. |
| 1.Upon receipt visually examine the culture for macroscopic evidence of any microbial contamination. |
| Using an inverted microscope (preferably equipped with phasecontrast optics), carefully check for any evidence of microbial contamination. Also check to determine if the majority of cells are still attached to the bottom of the flask? during shipping the cultures are sometimes handled roughly and many of the cells often detach and become suspended in the culture medium (but are still viable). |
| 2.If the cells are still attached, aseptically remove all but 5 to 10 mL of the shipping medium. The shipping medium can be saved for reuse. Incubate the cells at 37°C in a 5% CO2 in air atmosphere until they are ready to be subcultured. |
| 3.If the cells are not attached, aseptically remove the entire contents of the flask and centrifuge at 1000rpm for 5 to 10 minutes. Remove shipping medium and save. Resuspend the pelleted cells in 10 mL of this medium and add to 25 cm2 flask. Incubate at 37°C in a 5% CO2 in air atmosphere until cells are ready to be subcultured. |
III. Subculturing Procedure
| Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes. |
| 1.Remove and discard culture medium. |
| 2.Briefly rinse the cell layer with 0.25% (w/v) Trypsin0.53 mM EDTA solution to remove all traces of serum that contains trypsin inhibitor. |
| 3.Add 2.0 to 3.0 mL of Trypsin-EDTA solution to flask and observe cells under an inverted microscope until cell layer is dispersed (depend on the batch of trypsin). |
| Note: To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal. |
| 4.Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting. |
| 5.Add appropriate aliquots of the cell suspension to new culture vessels. |
| 6.Incubate cultures at 37°C. |
| Note: Subculture at 85% of confluence (about every 6 to 7 days). |
| Subcultivation Ratio: A subcultivation ratio of 1:4 to 1:5 is recommended |
| Medium Renewal: 2 to 3 times per week |
IV. Cryopreservation Procedure
| Volumes used in this protocol are for 75 cm2 flask; proportionally reduce or increase amount of dissociation medium for culture vessels of other sizes. |
| 1.Remove and discard culture medium. |
| 2.Briefly rinse the cell layer with 0.25% (w/v) Trypsin0.53mM EDTA solution to remove all traces of serum which contains trypsin inhibitor. |
| 3.Add 2.0 to 3.0 mL of Trypsin-EDTA solution to flask and observe cells under an inverted microscope until cell layer is dispersed (usually within 1 to 10 minutes). |
| Note: To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal. |
| 4.Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting. |
| 5.To remove trypsin-EDTA solution, transfer cell suspension to a centrifuge tube and spin at approximately 1000rpmfor 5 to10 minutes. |
| 6.Discard supernatant and resuspend cells in cryopreservation medium. |
| 7.Transfer the cells into Cryogenic Vials, 1ml/vial. |
| 8.Frozen the cells in cryogenic container (Nalgene #5100-0001). |
V. Database
| Mutation: |
| ||||||||
| Expression: | PRDX6, UPK2, CAPN6 | ||||||||
|
|
VI. References
| 1.PubMed=10737795; DOI=10.1073/pnas.97.7.3352; Rowan A.J., Lamlum H., Ilyas M., Wheeler J., Straub J., Papadopoulou A., Bicknell D., Bodmer W.F., Tomlinson I.P.; "APC mutations in sporadic colorectal tumors: a mutational 'hotspot' and interdependence of the 'two hits'."; Proc. Natl. Acad. Sci. U.S.A. 97:3352-3357(2000). |
| 2.PubMed=20606684; DOI=10.1038/sj.bjc.6605780; Bracht K., Nicholls A.M., Liu Y., Bodmer W.F.; "5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency."; Br. J. Cancer 103:340-346(2010). |
| 3.PubMed=24755471; DOI=10.1158/0008-5472.CAN-14-0013; Mouradov D., Sloggett C., Jorissen R.N., Love C.G., Li S., Burgess A.W., Arango D., Strausberg R.L., Buchanan D., Wormald S., O'Connor L., Wilding J.L., Bicknell D., Tomlinson I.P.M., Bodmer W.F., Mariadason J.M., Sieber O.M.; "Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer."; Cancer Res. 74:3238-3247(2014). |
| 4.PubMed=25485619; DOI=10.1038/nbt.3080; Klijn C., Durinck S., Stawiski E.W., Haverty P.M., Jiang Z., Liu H., Degenhardt J., Mayba O., Gnad F., Liu J., Pau G., Reeder J., Cao Y., Mukhyala K., Selvaraj S.K., Yu M., Zynda G.J., Brauer M.J., Wu T.D., Gentleman R.C., Manning G., Yauch R.L., Bourgon R., Stokoe D., Modrusan Z., Neve R.M., de Sauvage F.J., Settleman J., Seshagiri S., Zhang Z.; "A comprehensive transcriptional portrait of human cancer cell lines."; Nat. Biotechnol. 33:306-312(2015). |